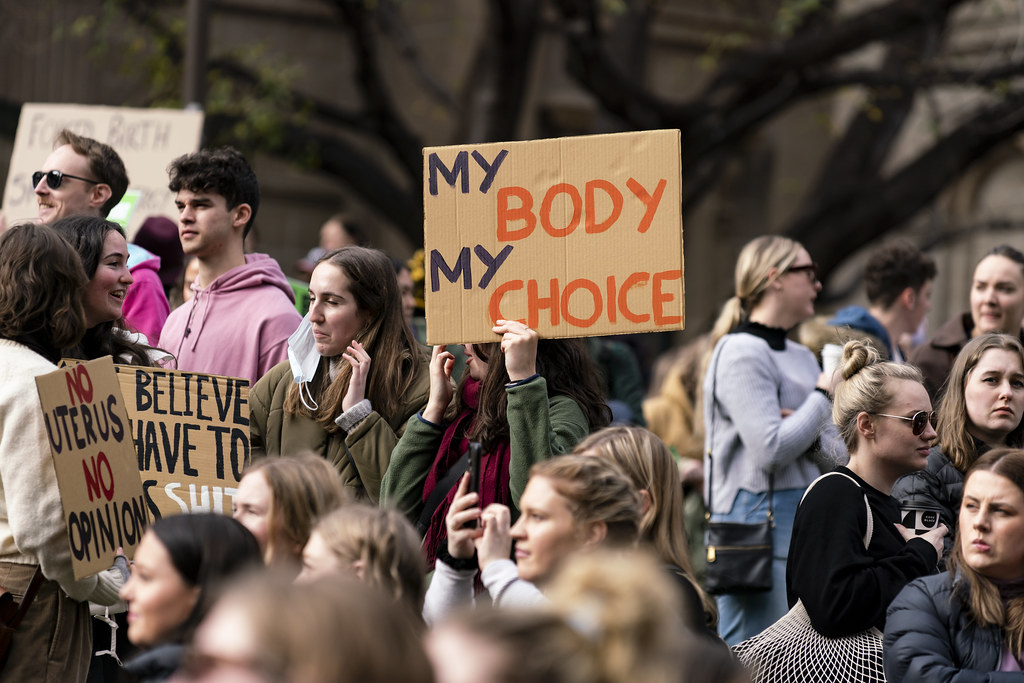Coronavirus Vaccine
December 19, 2020
The coronavirus vaccine is already controversial before it has even been released in the United States. Many Americans are declaring that they will not take the vaccine even if it’s easily obtainable. Vaccines take years of testing and research before they’re allowed to be sold to the public. Scientists have been racing to produce a safe vaccine. Researchers are testing 58 vaccines on humans and at least 86 preclinical vaccines on animals.
The first vaccine safety trials for humans started in March and now 13 vaccines have made their way to the final stages of testing before being released. There are five stages to the testing process for vaccines. Preclinical testing is when scientists test the vaccine on cells and then animals like mice or monkeys to see if it produces an immune response. In phase one, scientists give the vaccine to a small group of people to see if it stimulates the immune system. In phase two, they give the vaccine to hundreds of people split into groups by age to see if it affects older people differently than younger people. This trial is to see if the vaccine is safe and to see if it stimulates the immune system. Phase three is when scientists give the vaccine to thousands of people and wait to see how many people get infected. In this trial they measure the efficiency rate to see if the vaccine protects against the coronavirus. The FDA told vaccine makers that they needed to see proof of a 50% efficiency rate before they could be released. Phase three makes it easier to see common side effects of the vaccine because of the large number of people taking it, which they wouldn’t have been able to see in earlier trials. The next step is early or limited approval; so far China and Russia have approved vaccines without finding out what the results were for phase three. The final step is approval, which is when regulators in each country review the trial results and decide if the vaccine is good enough to be released to the public. During a pandemic, however, vaccines can receive emergency use authorization before getting formal approval from other countries. Once a vaccine is approved, researchers still monitor the people that take it to make sure that it’s safe and effective.
On November 9th, the New-York-based company Pfizer and German company BioNTech presented preliminary data showing that their vaccine was over 90 percent effective. BioNTech and Pfizer partnered up so they could further the research process. They started trials in May, and they realized that the vaccine caused volunteers to produce antibodies against the virus as well as immune cells called T cells that respond to the virus. In July the companies had announced that they have begun phases two and three with 30,000 volunteers worldwide but expressed that they wanted to expand their U.S. trial to 44,000 people. On December 2nd, the United Kingdom gave emergency authorization to Pfizer and BioNTech’s vaccine.